Which Name Is Used to Describe the Following Molecular Shape
E it is equal to the number of atoms in the molecule. Describe the molecular geometry.

Molecular Geometry Boundless Chemistry
Heres what I get.
. The periodic table can be used to calculate the molar mass of any substance 2. D it is equal to the sum of the atomic weights. B2O3 3C 3Cl2 2BCl3 3CO.
Two strands of nucleotides bonded together at their bases forming a. Carbon colorred2 This atom has three atoms directly attached and no lone. If all molecules were linear then life as we know it would not exist.
Look at the chart you just finished filling out. BCl3 Lewis Structure Molecular Geometry and Hybridization. Molecular shape also influences the boiling point and melting point of molecules.
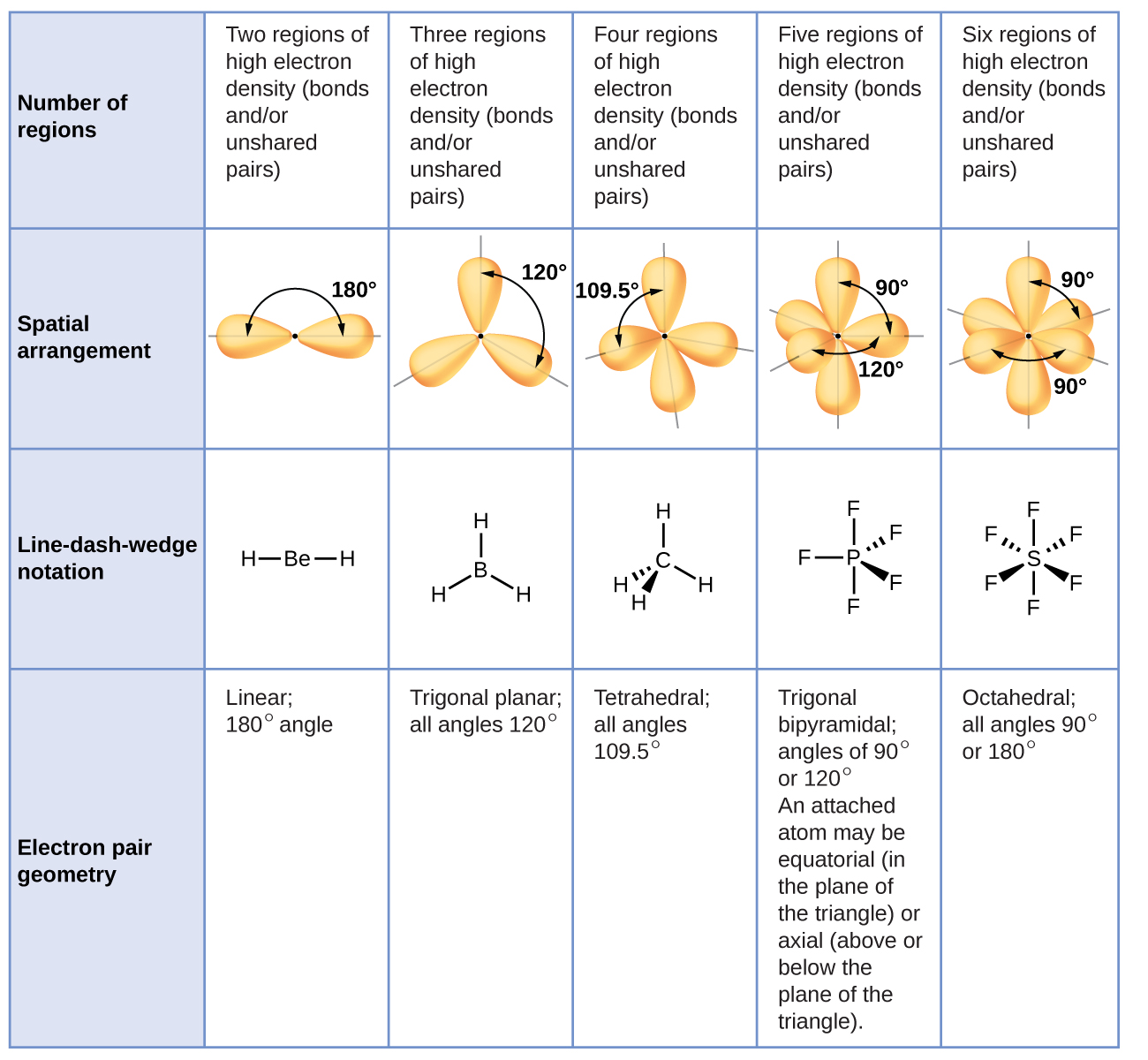
The term that describes the shape of the DNA molecule is that it is a very long double helical bases structure. Molecular Geometries Where Central Atom Has Lone Pairs Continued Original Shape without Lone Pairs of Outer Atoms of Lone Pairs General Formula Molecular Geometry Name trigonal planar AB3 2 1 AB2E bent or angular 3 1 AB3E. In this example we can draw two Lewis structures that are energetically equivalent to each other that is they have the same types of bonds and the same types of formal charges on all of the structuresBoth structures 2 and 3 must be used to represent the molecules structureThe actual molecule is an average of structures 2 and 3 which are called resonance structures.
A single strand of nucleotides bonded together forming a long. The total number of shared and unshared electron pairs around the central atom gives the electron-domain geometry. We can use VESPR theory to predict a trigonal pyrimidal shape for the molecule PF_3 because of its AX_3E status.
Molecular Geometry Boundless Chemistry Chemical Bonding Molecular Shapes And Vsepr Theory Britannica Molecular Geometry Boundless Chemistry Share. A double ring structure is found in which of the following bases. The VSEPR shape of the molecule PF_3 is trigonal pyrimidal.
The five molecular geometries are linear trigonal planar bent tetrahedral trigonal pyramidal. Trigonal bypyramidal molecular structure. This theory basically says that bonding and non-bonding electron pairs of the central atom in a molecule will repel push away from.
No comments for What Name Is Used to Describe the Following Molecular Shape Post a Comment. Its electron geometry and its molecular geometry are both tetrahedral as in methane. If the shape of a molecule is linear the polarity is _____.
In which the strands run in an anti parallel manner. Popular Total Pageviews Powered by Blogger Labels 15k 360 Aaps Airlines Alamat Apa Are Autodesk Best. The phrase electron domain is used in discussions of molecular geometry to mean either a lone pair or a bond on the central atom of a molecule.
The various molecular geometries for these types of molecules are shown in tables and described on the following pages. Laminiaduo7 and 12 more users found this answer helpful. The molar mass of a molecular substance is the mass per mole of its molecules.
If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal eg. Corresponding trihalides of boron are produced during the reaction of boron with halogens. 32 Molecular shape ESBM9 Molecular shape the shape that a single molecule has is important in determining how the molecule interacts and reacts with other molecules.
Anonymous C All electron groups are bonding pairs so PF 5 is designated as AX 5. Molecular geometry on the other hand depends on not only on the number of electron groups but also on the number of lone pairs. Total up the number of electron domains for each row in the chart and write it.
What is the correct name. Uses of BCl3 are immense. This means that 1 mole of oxygen atoms has a mass of 1600 g 3.
Notice that this gives a total of five electron. Write at least 2 sentences about each. You may also use your Molecular Geometry Charts if necessary PBr3.
When the electron groups are all bond pairs they. B it is equal to the number of covalent bonds in the molecule. To describe the first double-stranded molecule located in the cells nucleus.
Select all the statements that correctly describe the bonding and geometry in the polyatomic ion SeCl5-. VESPR stands for valence shell electron pair repulsion. The molar mass of oxygen is 1600 g.
A it is measured in atomic mass units. Molecular geometry is tetrahedral eg. Trigonal bypyramidal molecular structure.
Which of the following makes proteins from the RNA strand in the Cytoplasm. Forming a sphere. Boron trichloride is a molecule that can be industrially produced by chlorinating boron oxide and carbon directly at 501C.
Name the following compound. Molecular geometry is the name of the geometric shape used to describe the shape of a molecule. We must first draw the Lewis structure of acetic acid.
Name the following compound. Use the molecular formula to draw the Lewis structure. A The central atom P has five valence electrons and each fluorine has seven valence electrons so the Lewis structure of PF 5 is Figure PageIndex6.
Describe each of the following 5 properties of metals. If there are two bond pairs and two lone pairs of electrons the molecular geometry is angular or bent eg. As seen in the interactive the protein is made of smaller monomer molecules.
C it is useful for calculating concentrations.

Molecular Geometry Boundless Chemistry

Comments
Post a Comment